Trona Ore Mining
Chemical Reactions
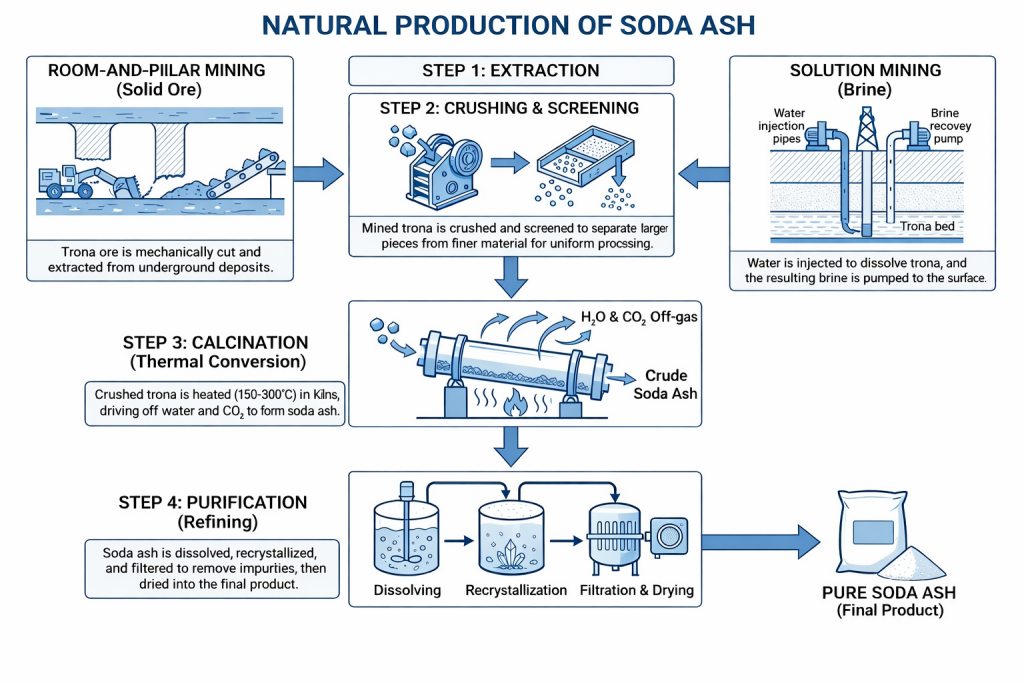
Trona (Na₂CO₃·NaHCO₃·2H₂O) is extracted from underground deposits using room-and-pillar mining or solution mining, depending on geological conditions.
Mined trona is crushed and screened to remove insoluble impurities such as shale and clay.
Purpose:
- Increase ore purity
- Improve thermal conversion efficiency
Crushed trona is heated to decompose sodium bicarbonate and remove water of crystallization, producing soda ash.
Reaction:
Na₂CO₃·NaHCO₃·2H₂O → Na₂CO₃ + CO₂ + 2 H₂O
This step directly yields soda ash light.
In higher-grade applications, calcined material may be dissolved, clarified, and recrystallized to improve purity.
The soda ash is dried and classified to meet particle size and bulk density specifications (light or dense grades).
Overall Net Reaction
Net transformation:
Trona → Na₂CO₃ + CO₂ + H₂O
No external reagents (ammonia, lime, or salt) are required.
Raw Materials and Source
Trona ore is a naturally occurring mineral used as the primary raw material for natural soda ash production. Its chemical composition is Na₂CO₃ · NaHCO₃ · 2H₂O, which means it naturally contains sodium carbonate, sodium bicarbonate, and water in a single mineral form. Trona is extracted as a solid mineral from underground deposits using conventional mining methods, then typically processed through crushing, heating (calcination), and purification to produce soda ash (sodium carbonate) in the required commercial grade.

By Products
In trona ore mining, the main byproducts are carbon dioxide (CO₂) and water, usually released as steam and later collected as condensed water. Depending on the plant setup, the CO₂ may be reused inside the process, captured for other industrial uses, or released through controlled emission systems. The water is typically condensed and reused as process water or utility water to support operations.

