Solution Mining
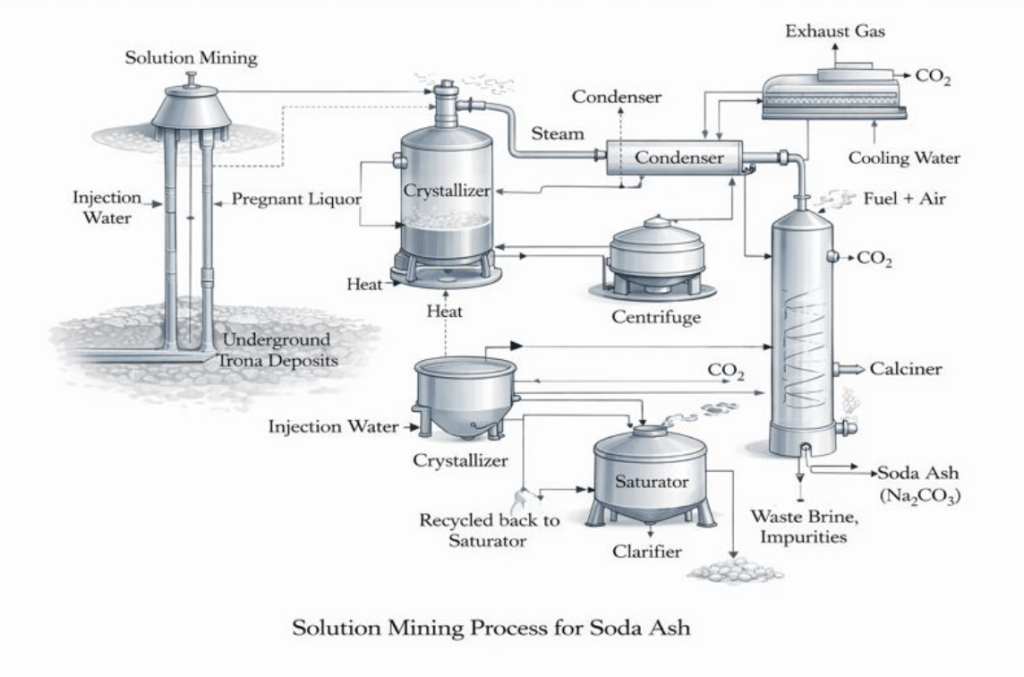
Process Overview
Injection and production wells are drilled into the trona-bearing formation. Caverns are created to allow controlled dissolution of the ore.
Hot water or recycled sodium carbonate solution is injected to dissolve trona underground, forming a sodium carbonate rich brine.
Dissolution reaction:
Na₂CO₃·NaHCO₃·2H₂O (s) → Na₂CO₃ (aq) + NaHCO₃ (aq) + 2 H₂O
The carbonate rich brine is pumped to the surface and clarified to remove insoluble solids and geological impurities.
The clarified brine is cooled or evaporated to selectively crystallize sodium bicarbonate or sodium carbonate hydrates, depending on operating conditions.
Example crystallization step:
NaHCO₃ (aq) → NaHCO₃ (s)
The crystallized sodium bicarbonate is calcined to produce soda ash light.
Reaction:
2 NaHCO₃ → Na₂CO₃ + CO₂ + H₂O
Released CO₂ may be vented or partially reused within the process.
The soda ash is dried, milled, and classified to meet particle size and bulk density specifications.
Overall Net Reaction
Net transformation:
Na₂CO₃·NaHCO₃·2H₂O → Na₂CO₃ + CO₂ + H₂O
Raw Materials and Source
In solution mining, the primary feedstock is trona contained in underground ore deposits, with the composition Na₂CO₃ · NaHCO₃ · 2H₂O. Instead of mining the ore as a solid, hot water or recycled process water is injected underground to dissolve the trona, forming a carbonate-rich brine. This brine is then pumped to the surface for further processing, where soda ash is recovered through controlled evaporation, crystallization, and calcination.

By Products
In solution mining, the byproducts are also mainly CO₂ and water. CO₂ is usually handled the same way as in trona ore mining: it can be recycled back into the process, captured, or released with emissions control, depending on the facility. Water recovery is especially important in this method, so the condensed water is commonly recovered and reused, often supporting the brine and solution circulation loops used in the mining and processing stages.

