Hou Process
Chemical Reactions
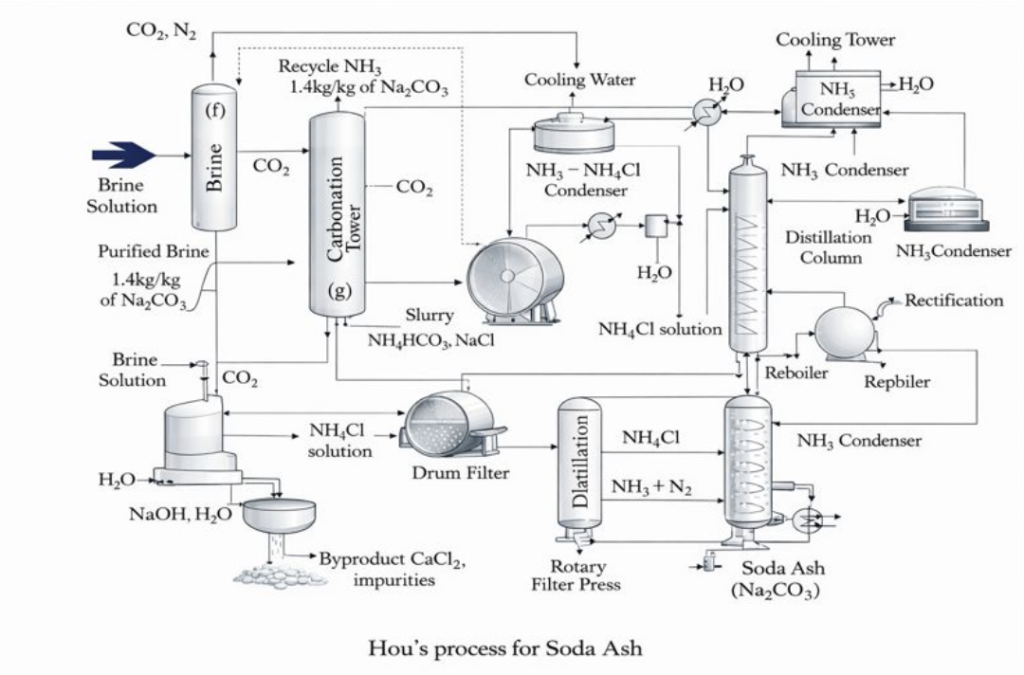
Purified sodium chloride (NaCl) brine is prepared to eliminate Ca²⁺ and Mg²⁺ impurities that could affect crystallization efficiency.
Ammonia (NH₃) is absorbed into the brine to form ammoniated brine, enabling controlled carbonation and crystallization.
Carbon dioxide (CO₂) is introduced into the ammoniated brine, precipitating sodium bicarbonate (NaHCO₃).
Reaction:
NaCl + NH₃ + CO₂ + H₂O → NaHCO₃ ↓ + NH₄Cl
The sodium bicarbonate is filtered and calcined to produce soda ash light.
Reaction:
2 NaHCO₃ → Na₂CO₃ + CO₂ + H₂O
CO₂ is recycled back into the carbonation step.
Instead of decomposing ammonium chloride, the Hou Process further cools and concentrates the solution to crystallize NH₄Cl as a solid product.
Reaction (crystallization):
NH₄Cl (aq) → NH₄Cl (s)
This eliminates the need for lime-based ammonia recovery.
Only minor ammonia makeup is required to compensate for system losses, improving raw material efficiency.
Overall Net Reactions
Main soda ash formation:
2 NaCl + CO₂ + NH₃ + H₂O → Na₂CO₃ + NH₄Cl
(NH₃ is partially recycled but also locked into NH₄Cl product)
Raw Materials and Source
The Hou process uses salt brine (sodium chloride, NaCl) as its primary raw material, typically sourced from seawater, salt lakes, or underground salt deposits. Ammonia (NH₃) is an essential circulating agent that enables the formation of soda ash and is largely recovered and reused within the process. Carbon dioxide (CO₂) is supplied from limestone calcination or industrial flue gas and is used to convert the ammoniated brine into soda ash precursors. Water is required for brine preparation and process circulation, while energy supports heating and calcination stages. Compared to the traditional Solvay process, the Hou process is designed to utilize these raw materials more efficiently and to recover ammonium chloride as a valuable co-product, improving overall resource efficiency.
By Products
The Hou process produces ammonium chloride (NH₄Cl) as a key co-product, along with CO₂. NH₄Cl is usually recovered by crystallizing it from the remaining solution and then drying it into a saleable form. It is most commonly sold as fertilizer-grade ammonium chloride, but it can also be used in dry cell batteries, metal treatment and galvanizing, and other industrial applications depending on the grade. Similar to the Solvay process, CO₂ is typically recycled back into the process, and any excess may be captured or released depending on the plant setup.

