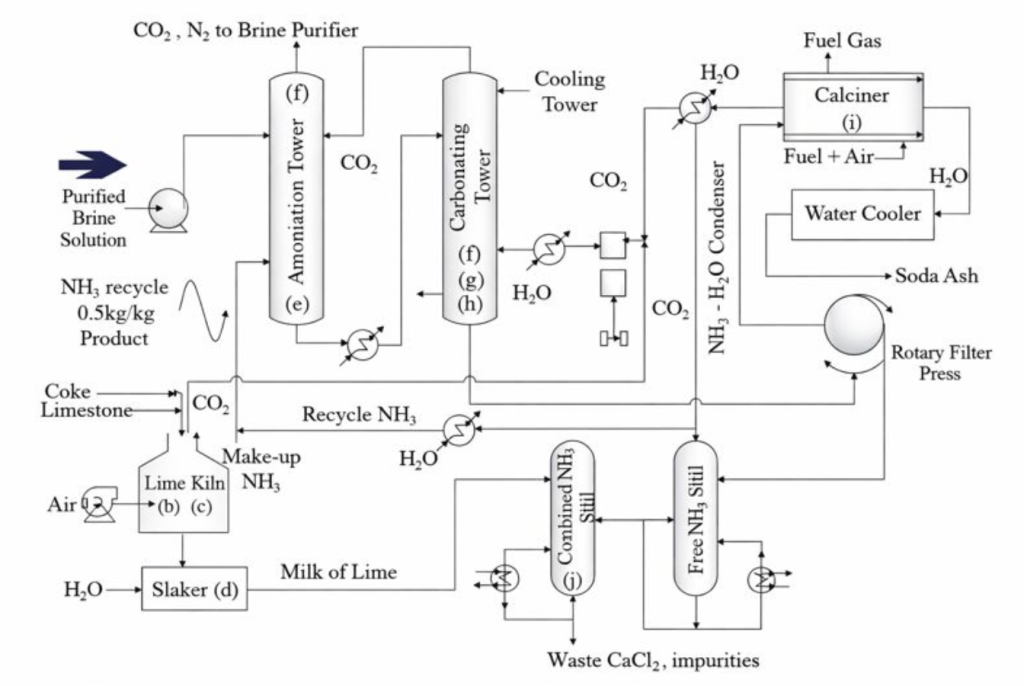
Synthetic Process
Chemical Reactions
Purified sodium chloride (NaCl) brine is prepared to remove calcium and magnesium impurities that would interfere with downstream precipitation.
Ammonia (NH₃) is dissolved into the purified brine to increase alkalinity and enable selective precipitation in the carbonation stage.
Reaction (simplified):
NaCl + NH₃ + H₂O → Ammoniated brine
Carbon dioxide (CO₂), generated from limestone calcination, is bubbled through the ammoniated brine. Sodium bicarbonate (NaHCO₃), which has low solubility, precipitates out.
Key reaction:
NaCl + NH₃ + CO₂ + H₂O → NaHCO₃ ↓ + NH₄Cl
The sodium bicarbonate precipitate is filtered and heated (calcined) to form soda ash light (sodium carbonate).
Reaction:
2 NaHCO₃ → Na₂CO₃ + CO₂ + H₂O
Released CO₂ is recycled back to the carbonation stage.
The remaining ammonium chloride solution is reacted with lime (Ca(OH)₂) to regenerate ammonia for reuse.
Reaction:
2 NH₄Cl + Ca(OH)₂ → 2 NH₃ ↑ + CaCl₂ + 2 H₂O
Limestone is thermally decomposed to supply CO₂ and lime.
Reaction:
CaCO₃ → CaO + CO₂
CaO + H₂O → Ca(OH)₂
Overall Net Reaction (Simplified)
Net reaction:
2 NaCl + CaCO₃ → Na₂CO₃ + CaCl₂
Ammonia is fully recycled and does not appear in the final reaction balance.
Raw Materials and Source
The Synthetic process uses salt brine (sodium chloride, NaCl) as the primary raw material, which is typically sourced from seawater, salt lakes, or underground salt deposits. Limestone (calcium carbonate, CaCO₃) is another key input and is obtained from natural limestone quarries; it serves as the source of carbon dioxide used in the process. Ammonia (NH₃) is used as a circulating agent to enable the chemical reactions and is largely recovered and reused within the system rather than consumed. Water and energy are also required as supporting inputs. Together, these raw materials allow soda ash to be produced through a controlled chemical route, making the Solvay process one of the most widely used synthetic methods globally.

By Products
In the Synthetic process, the main byproduct is calcium chloride (CaCl₂), along with some CO₂. Calcium chloride is usually concentrated into a liquid solution, and in some cases it is further processed into flakes or pellets if the market requires it. It is widely used for road de-icing, dust control, and oil and gas drilling fluids, and in some regions it is also used as a construction additive. The CO₂ produced is often reused in the carbonation step, while any extra CO₂ may be captured or released, depending on how the plant is designed.

